Taking Care of Patient With LVAD and CRT-D Undergoing Total Hip Arthroplasty
Yifan Bu, MD
Published April 18, 2025 | Clinics in Medical Education
Issue 6 | Volume 1 | April 2025
A 78-year-old male has a past medical history of transthyretin-mediated amyloid non-ischemic cardiomyopathy with EF of 10% s/p HM3 LVAD implant in 2021. He also has chronic atrial fibrillation and VT s/p ICD with CRT-D upgrade in 2021. He now presented for a left hip arthroplasty. The patient was admitted for bridging his warfarin to heparin before surgery. The Anesthesia plan was general anesthesia with a-line monitoring.
What is a ventricular assist device (VAD)?
Ventricular assist devices (VADs) are mechanical devices inserted to assist cardiac function by offloading part or all of the pumping responsibilities from the ventricle. Placement of a VAD can be done on the left side of the heart to assist in left ventricular function (LVAD) or on the right side of the heart to help with the right ventricle (RVAD). The presence of both an LVAD and an RVAD is referred to as biventricular support (BiVAD). A number of different VAD constructs exist, with major differentiators being pulsatile versus continuous flow, extracorporeal versus intracorporeal, degree of assistance provided, ability to help the left or right sides, and the length of time it can be used.
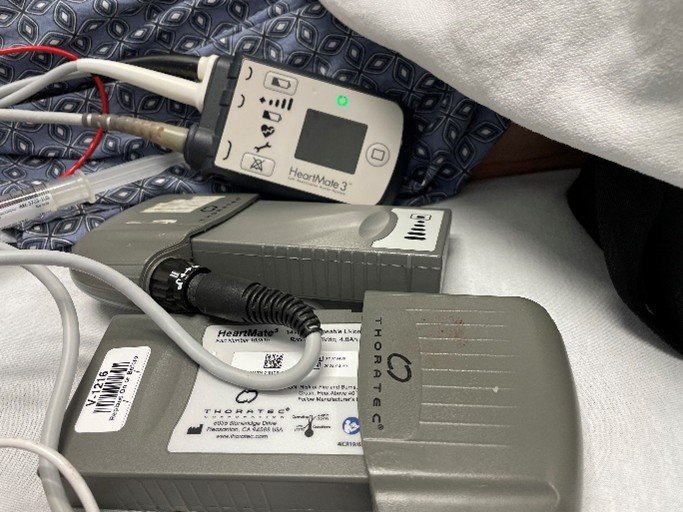
What type of LVAD does this patient have? What characteristics does this type of LVAD have?
This patient has a THORATEC Heart Mate 3. It is a continuous flow LVAD. Continuous flow VADs are valveless pumps that use a magnetic field to rapidly spin a single impeller supported by mechanical or magnetic, or hydrodynamic bearings. Patients may not exhibit arterial pulsatility due to the continuous blood flow provided by the VAD, but they may still have sufficient residual or recovered ventricular function to generate intrinsic pulsatile flow. Given the continuous-flow pump characteristics, measuring the mean arterial pressure (MAP) is the most reliable measure of perfusion pressure and is the standard of care for VAD patients.
What is our institutional protocol when taking care of patients with VADs? Who should be monitoring the VADs function in pre-op holding, OR, and PACU?
Our department has a detailed protocol discussing how to take care of patients with VADs, which can be found at C8Health: WORKFLOW FOR PERIOPERATIVE CARE OF THE PATIENT WITH A DURABLE LEFT VENTRICULAR ASSIST DEVICE (VAD).
Pre-op: Patients with VADs are knowledgeable regarding the care of their devices. If the patient is considered VAD competent according to BIDMC’s level of education, he or she will manage the device in the preoperative holding area prior to receiving sedation. If a patient is not VAD competent due to current medical or training status, a member of the VAD team will remain immediately available in the preoperative area and be responsible for device monitoring. If a patient requires sedation or is having an arterial line placed (without sedation), he or she is no longer competent to monitor his or her device. The perfusionist assigned to the case will assume the monitoring responsibility.
Intraoperatively, the device monitoring will be provided by a member of the perfusion team. The perfusionist will remain either physically present or immediately available during the entirety of the case.
Procedural recovery area:
In the recovery area, the VAD competent RN/NP is responsible for monitoring the VAD and will continue to perform Doppler blood pressure measurements (if without a-line monitoring). Any concerns regarding VAD function as it relates to the patient clinically will be communicated to the recovery room nurse and the advanced heart failure attending physician if warranted.
One day prior to surgery, we contacted both the perfusion team and the EP team to discuss perioperative LVAD and CRT-D management plans.
For LVAD, we confirmed that a perfusionist was assigned to this case and would meet the patient in the pre-op holding area before the a-line placement.
For CRT-D, EP team was contacted to reprogram his pacemaker settings. Based on patient’s recent (02/2025) CRT-D interrogation report, the device was programmed as mode DDD 70-130 w/ tachy therapies on. EP team reprogrammed it to DOO 70 w/ tachy therapies off one night prior to surgery. CRD-T response with magnet is: inhibits therapies, with no effect on the pacemaker. Since the incision site was infra-groin, a decision was made not to use the magnet to suspend the ICD function but to have one available.
In pre-op, we evaluated the LVAD function, drive line, dressing and extra supplies together with the perfusionist. Due to the continuous LVAD flow, obtaining blood pressure readings from non-invasive blood pressure cuffs and oxygen saturations from pulse oximeters may not be feasible or reliable.

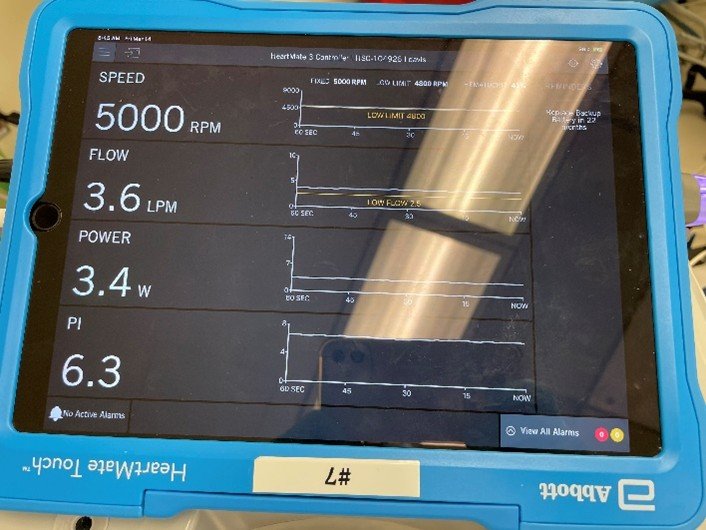
What are the key parameters of this LVAD device?
- Pump speed: measured in RPM and relates to how fast the impeller is spinning. This is the only variable programmed by the operator.
- Flow: measured in liters per minute (LPM). This correlates with pump speed, and is dependent on the pressure differential (also known as head pressure) across the pump. This pressure differential is in turn dependent on the ventricular chamber pressure (i.e., preload), vascular tone (i.e., afterload), and factors affecting blood viscosity (e.g., hematocrit).
- Power: the amount of power the VAD consumes to continuously run at a set speed. A sudden or gradual increase in the power can indicate obstruction or thrombus inside the VAD.
- Pulsatility Index (PI): a measure of the pressure differential within the VAD pump and indicates the proportion of cardiac output provided by the native heart versus the device. In addition to the left ventricular contractility, the PI of an LVAD is affected by the patient’s volume status and right ventricular function.
After an uneventful induction with lidocaine, etomidate, fentanyl, and rocuronium, the patient was on 0.7% MAC of sevoflurane. The LVAD monitor showed the parameters below:
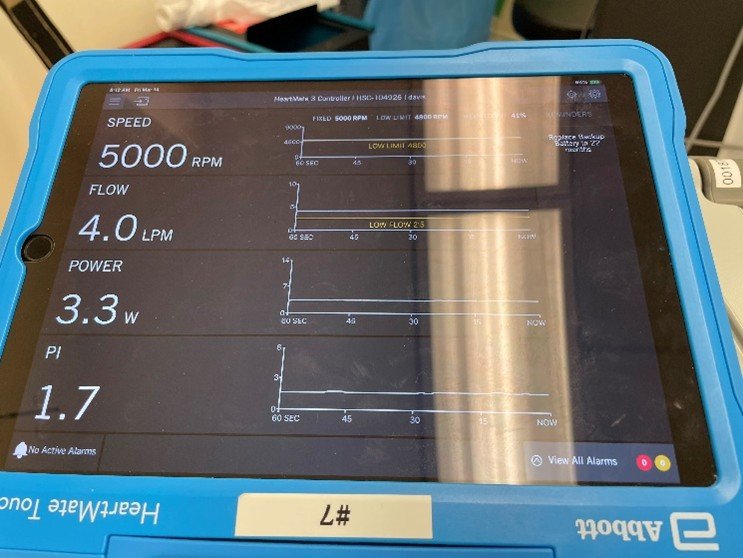
Compared with the parameters before induction, what happened to the patient, and how should it be treated?
The patient had a decreased PI from his baseline, slightly increased Flow, unchanged Power and RPM
Interpretation: vasodilation or hypovolemia. The pump is “starving” for volume, resulting in a drop in PI (less pulsatility because the left ventricle is not filling adequately).
Possible Causes: vasodilation under anesthesia.
Management / Treatment: titrated fluid bolus, adjusting anesthetic depth.
What are other possible parameter changes that can happen intraoperatively, and how to interpret them?
Scenario 1. High PI, High Flow, Normal Power, Normal RPM
- Interpretation: increased volume return or improved native LV contractility
- Possible Causes: potential hypervolemia caused by over-resuscitation with fluids; vasoconstriction or increased venous return (e.g., after administration of vasopressors).
- Management / Treatment: check for signs of fluid overload (lung auscultation for crackles, patient’s oxygenation), then optimize fluid management; if significantly hypervolemic, consider diuretics or adjusting fluid administration. Monitor hemodynamics to ensure no excessive increase in LV pressures or risk of right heart failure.
Scenario 2. Rising Power, Steady or Slightly Decreasing Flow, Normal/Stable RPM, Unchanged PI
- Interpretation: possible pump thrombus or obstruction. The motor works harder to maintain the same speed in the presence of an obstruction (thrombus forming in the pump or partial inlet/outlet obstruction). If the power continues to rise without obvious hemodynamic changes, suspect a device-related issue.
- Possible Causes: pump thrombosis (most common suspicion). Kink or obstruction in the outflow graft. Clot formation near the inflow cannula.
- Management / Treatment: evaluate device function urgently in conjunction with the LVAD team. Anticoagulation check: Ensure therapeutic anticoagulation and/or start intravenous heparin if not contraindicated. Consider imaging (e.g., TEE) to evaluate inflow cannula, outflow graft, and cardiac function.
- Possible need for thrombolysis or surgical intervention if a pump thrombus is confirmed.
Scenario 3. Low PI, Sudden Decrease in Flow, Drop in Power, Unchanged RPM
- Interpretation: suction event or severe underfilling of the LV. If the left ventricle collapses around the inflow cannula (due to inadequate preload), the pump cannot maintain flow.
- Possible Causes: acute hypovolemia or sudden reduction in venous return. Excessive vasodilation or abrupt shift in fluid distribution.
- Management / Treatment: stop or reduce ongoing fluid loss (identify surgical bleeding or check if there’s a sudden drop in preload). Administer fluid bolus to restore preload. Consider lowering the RPM temporarily if suction alarms are triggering. Re-check the entire circuit (no disconnection or kinking).
REFERENCES
1. WORKFLOW FOR PERIOPERATIVE CARE OF THE PATIENT WITH A DURABLE LEFT VENTRICULAR ASSIST DEVICE (VAD)
2. HFSA/SAEM/ISHLT Clinical Expert Consensus Document on the Emergency Management of Patients With Ventricular Assist Devices. Givertz MM, DeFilippis EM, Colvin M, et al. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation. 2019;38(7):677-698.
3. Saeed, D., Feldman, D., El Banayosy, A., Birks, E., Blume, E., Cowger, J., Hayward, C., Jorde, U., Kremer, J., MacGowan, G. and Maltais, S., 2023. The 2023 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: a 10-year update. The Journal of Heart and Lung Transplantation, 42(7), pp.e1-e222.
