Understanding Atrial Fibrillation: A Brief Educational Review
Mona Hedayat, MD
Published December 2, 2024 | Clinics in Medical Education
Issue 4 | Volume 1 | November 2024
A 65-year-old male with a history of hypertension, diabetes mellitus, coronary artery disease (previous PCI 3 years ago), chronic kidney disease stage 3 and former smoker is undergoing elective laparoscopic cholecystectomy. During the procedure, the patient develops acute atrial fibrillation (AF) with a rapid ventricular response, leading to hemodynamic instability characterized by hypotension and signs of poor perfusion. The anesthesiologist checked ABG to rule out reversible causes (hypo or hyper-kalemia, acidosis), administered IV fluids cautiously to optimize preload and blood pressure and IV metoprolol to control rate, but the patient remains hemodynamically unstable.
According to 2023 guidelines from the American College of Cardiology (ACC), American Heart Association (AHA), American College of Chest Physicians (ACCP), and Heart Rhythm Society (HRS), emergency R-wave synchronized direct current (DC) electrical cardioversion is recommended for hemodynamically unstable patients with new-onset AF [1]. Caution is advised for patients with preoperative or unknown sinus node dysfunction or with patients receiving significant doses of rate controlling medications, as significant pauses can occur after DC cardioversion. For those patients, external pacing may be required and should be readily available [2].
The 2023 guidelines also provide specific recommendations regarding the need for echocardiography to find intracardiac thrombus prior to cardioversion in the context of managing AF [1].
- For patients with AF duration of ≥ 48 hours, a 3-week duration of uninterrupted therapeutic anticoagulation OR imaging evaluation to exclude intracardiac thrombus is recommended before elective cardioversion.
For patients with AF undergoing cardioversion, therapeutic anticoagulation should be established before cardioversion and continued for at least 4 weeks afterwards without interruption to prevent thromboembolism.
In patients with AF in whom cardio- version is deferred due to left atrial appendage (LAA) thrombus detect- ed on pre-cardioversion imaging, therapeutic anticoagulation should be instituted for at least 3 to 6 weeks, after which imaging should be repeated before cardioversion.
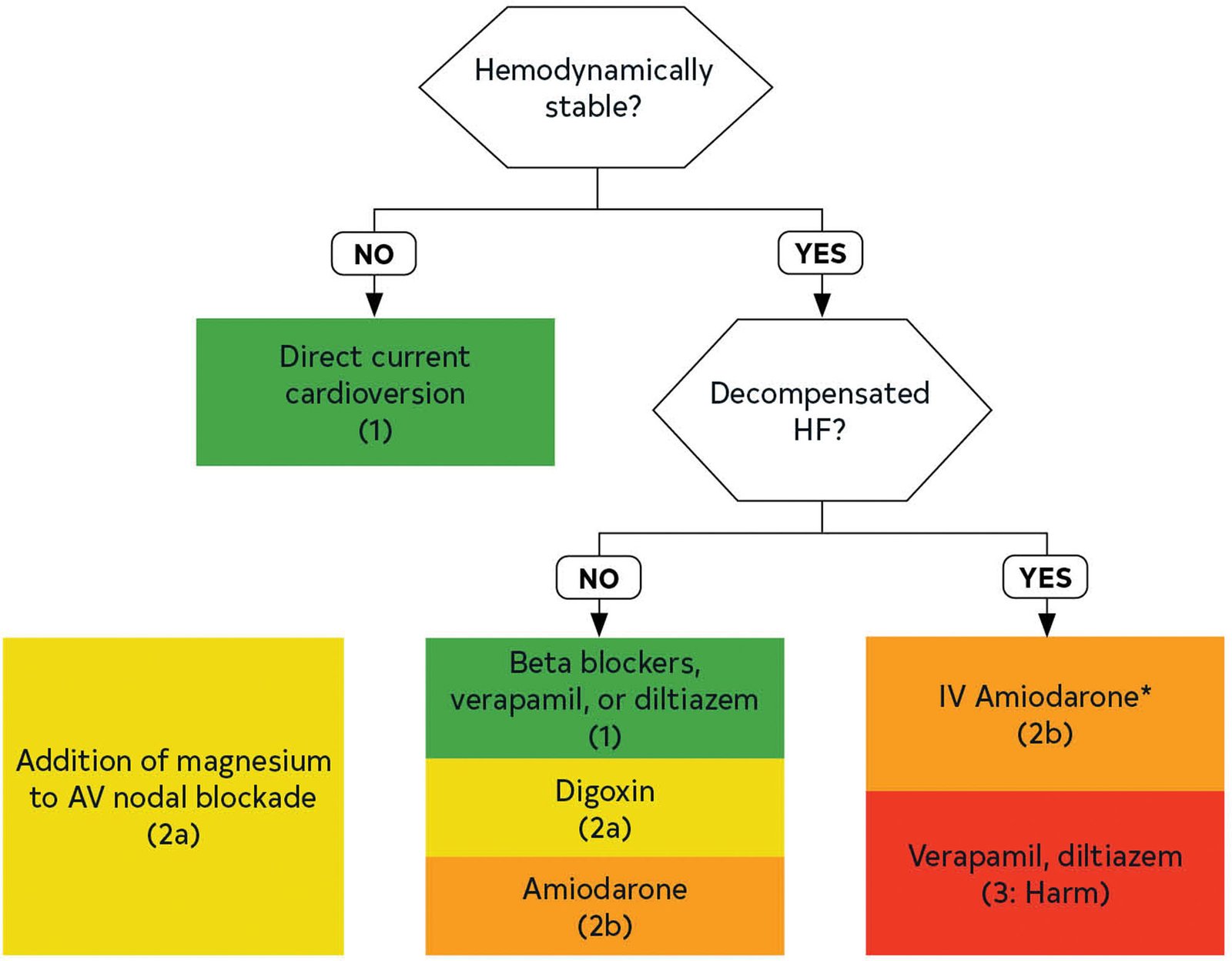
Figure 1
Acute Rate Control in AF With RVR.
* Contraindicated in patients with moderate-severe LV dysfunction regardless of decompensated HF.4 RVR: Rapid Ventricular Rate, HF; Heart Failure
For patients with reported AF duration of < 48 hours (not in the setting of cardiac surgery) and who are not on anticoagulation, pre-cardioversion imaging to exclude intracardiac thrombus may be considered in those who are at elevated thromboembolic risk (CHA2DS2-VASc score ≥ 2 or equivalent).
During the first 48 hours after the onset of paroxysmal AF, the need for anticoagulation before and after DC cardioversion may be based on the patient’s risk of thromboembolism (CHA2DS2-VASc score) balanced by the risk of postoperative bleeding [3].
In this case scenario, synchronized DC cardioversion was performed with an initial shock of 200 J, successfully restoring sinus rhythm with HR 78 bpm and BP 120/75. Following successful cardioversion, cardiac enzymes and BNP were sent to assess for myocardial ischemia or heart failure exacerbation. Postoperatively, intravenous heparin was administered to reduce the risk of thromboembolism. A cardiology consultation needs to be arranged for further management and to discuss long-term anticoagulation based on the patient’s CHA2DS2-VASc score.
For hemodynamically stable patients requiring acute rate control, here is the summary of the 2023 guideline recommendations [1]:
In patients with AF with rapid ventricular response who are hemodynamically stable, beta blockers or non-dihydropyridine calcium channel blockers (verapamil, diltiazem; provided that EF > 40%) are recommended for acute rate control.
In patients with AF with rapid ventricular response in whom beta blockers and non-dihydropyridine calcium channel blockers are ineffective or contraindicated, digoxin can be considered for acute rate control, either alone or in combination with the aforementioned agents.
In patients with AF with rapid ventricular response, the addition of intravenous magnesium to standard rate-control measures is reasonable to achieve and maintain rate control.
In patients with AF with rapid ventricular response who are critically ill and/or in decompensated HF in whom beta blockers and non-dihydropyridine calcium channel blockers are ineffective or contraindicated, intravenous amiodarone may be considered for acute rate control. Consider the risk of cardioversion and stroke when using amiodarone as a rate-control agent.
In patients with AF with rapid ventricular response and known moderate or severe LV systolic dysfunction with or without decompensated HF, intravenous non-dihydropyridine calcium channel blockers should not be administered.
REFERENCES
1. Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS Guidelines for the Prevention and Management of Perioperative Atrial Fibrillation and Flutter for Thoracic Surgical Procedures. Executive Summary. The Journal of Thoracic and Cardiovascular Surgery. 2014;148(3):772-91.
2. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/¬AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/¬American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24): e278-333.
3. Lucà F, Giubilato S, Di Fusco SA, et al. Anticoagulation in Atrial Fibrillation Cardioversion: What Is Crucial to Take Into Account. Journal of Clinical Medicine. 2021;10(15):3212.
4. Joglar, José A et al. “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines.” Circulation vol. 149,1 (2024): e1-e156. doi:10.1161/ CIR.0000000000001193
